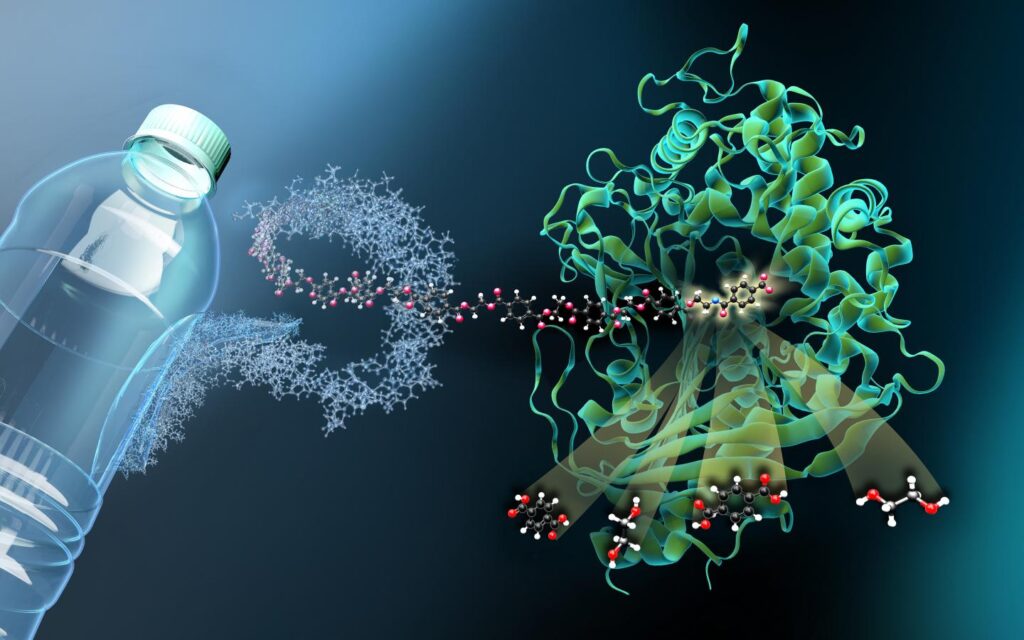
Plastic has grown inseparable from modern life since its emergence in the 1960s. Its versatility, durability, and low-production value allow it to be the leading material for commercial production, with manufacturers worldwide producing over 400 million metric tons of plastic annually (“Topic: Global Plastic Waste”). Consequently, plastic pollution has become a global environmental challenge, where approximately 85% of it reaches landfills and unregulated ecosystems every year (Barclay and Acharya 5). Traditional plastic waste management methods are now insufficient. Landfilling, for example, allows polymers to persist in the environment, polluting oceans and food chains. This resilience to organic decomposition is due to plastic’s hydrophobic nature, which prevents hydrolysis, the breaking down of chemical bonds with water (Barclay and Acharya 1). A predominant type of plastic, polyethylene terephthalate (PET), is used in “textile, packaging, and bottle-producing industries due to [its] high durability, elasticity, strength, and resistance to chemicals” (Deng et al. 2). Currently, PET recycling is mainly thermally treated, producing harmful carbon emissions and eliciting drastic health and environmental ramifications (Sevilla 1). One promising potential solution that avoids such concerns, highlighted by Amelia Barclay and K. Ravi Acharya from the Department of Life Sciences at the University of Bath, is the exploration of enzymes that degrade plastics into their organic fragments. These byproducts are then able to be repolymerized into new plastic or used as a carbon source for bacteria (2). An examination through the biochemical lens reveals to what extent plastic-degrading enzymes (PDEs) are a viable, or even preferred, solution for PET waste reduction.
Plastic is created by molecules of non-renewable fossil fuels, such as oil, gas, and coal, repeating to form strands of small organic molecules called polymers. Manufactured polymerization frequently requires high temperatures, intense pressure, and chemical modifications–all of which devastate microbes (TED-Ed 00:00:52-00:01:00). However, in their 2016 scholarly report, “A Bacterium That Degrades and Assimilates Poly(ethylene Terephthalate),” a group of Japanese researchers, led by Shosuke Yoshida, found that the bacterium Ideonella sakaiensis 201-F6 produces an enzyme capable of degrading PET, known as PETase, named after its affinity for PET (1196). Along with PETase, Ideonella sakaiensis 201-F6 also produces MHETase, which further breaks down the polymeric byproducts from PETase, converting the environmentally harmful PET into “environmentally benign monomers” (Yoshida 1196), the building units of polymers (Barclay and Acharya 1). Hydrolysis, achieved by breaking the ester bonds with water, results in the deconstruction of the polymer chain into organic acids and alcohols, leaving behind reusable monomers comparable to their raw material (Orlando et al. 2), and food for the bacterium (Barclay and Acharya 2).
Conveniently, PET is especially susceptible to enzyme degradation because of its signature hydrolysable ester bonds (Wei and Zimmerman 1309). Nurul Fatin Syamimi Khairul Anuar et al., from various bioscience faculties at the University of Technology in Malaysia, in their academic review, “An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation,” explain that this unique property allows PETase to degrade the plastic into mono terephthalate acid (MHET), which is then broken down by MHETase (13). This process results in terephthalate (TPA) and ethylene glycol (EG) that are carbon nutrients for bacteria (Yoshida 1197). However, this reduction to reusable units is unique to PET, leaving other plastics isolated from this solution. In contrast to Deng et al.’s claim that PET was the most prevalent form of plastic, Marco Orlando’s team from the Department of Biotechnology and Life Sciences at the University of Insubria, Italy, in their academic review, “Microbial Enzyme Biotechnology to Reach Plastic Waste Circularity: Current Status, Problems and Perspectives,” reports only approximately 7% of plastic waste is PET (1). The team emphasizes the importance of researching a broader range of enzymes to accommodate different plastics. Even among PET, when the polymer chains are closely packed to strengthen the plastic, creating crystalline PET, enzymes become inadequate (Barclay and Acharya 9). According to Barclay and Acharya, this nearly degradation resistant version of PET requires additional pre-treatment or elevated temperatures for the enzymes to function in their breakdown (9). To combat this concern, Maria Eduarda Sevilla et al. from the Faculty of Science and Engineering in Food and Biotechnology at the Technical University of Ambato, Ecuador, found in her research article that introducing specific PETase mutations increases the degradative capacity of PETase to include crystalline PET more than their wild type, or naturally occurring, counterparts (1). Her findings support Barclay and Acharya’s advocacy for guided evolution to advance favorable traits, with the goal of developing variants to enhance the catalytic efficiency of PDEs (2).
Nonetheless, when degradation is achieved by microscopic enzymes, the process is gradual and often incomplete. One way of accelerating depolymerization is by raising the temperature of the process, such as to 65-70℃, increasing polymer chain pliability (Çakar et al. 476). However, microbes cannot withstand such high temperatures. In support, a research group from State Key Laboratory of Biocatalysis and Enzyme Engineering School of Life Sciences at Hubei University, in their scholarly article, “Improving the Activity and Thermostability of PETase from Ideonella Sakaiensis Through Modulating its Post-Translational Glycan Modification,” assert the PETase wild type is “thermosensitive and loses most of its activity within 24 hours at 37℃,” limiting its industrial applications (Deng et al. 2). Hence, engineered variations of PETase emerge with higher thermostability, such as FAST-PETase, which “demonstrates elevated efficiency in degrading postconsumer-PET trays under 55°C” (Deng et al. 1). Additionally, Barclay and Acharya reaffirm that accommodation for PET substrates can enhance industrial use with the support of tools such as computational AI models that identify patterns for directed evolution of enzyme efficiency (9). With the help of modern technology and
biochemical engineering, PETase grows to increased efficiency and applicability, becoming a dominant solution to plastic waste reduction.
However, PETase is not the only PDE available. Before the discovery of PETase in 2016, cutinases, a subtype of esterase enzymes, have been identified to degrade cutin, a waxy polymer with fatty acids for a hydrophobic quality (Barclay and Acharya 1). For their shared ability to hydrolyze hydrophobic compounds, PETase and cutinase are often recognized as leading PDEs. In their 2023 review, “Biotechnological Plastic Degradation and Valorization Using Systems Metabolic Engineering,” Ga Hyun Lee and her team from various esteemed clean energy and biotechnology institutes in Seoul, Korea, compare PETase against various cutinases. While PETase and MHETase, both produced by Ideonella sakaiensis, worked together for a 100% degradation of PET in 30-37℃, the three different cutinases studied were only able to degrade a maximum of 90% of PET, even with heat pretreated PET (Son et al.; Tournier et al. qtd. in Lee 4). Thus, these findings prove the enzymes from Ideonella sakaiensis are the most effective biotechnological method for reducing plastic waste.
Overall, PDEs are an exceedingly viable solution to limiting the accumulation of plastic waste in the world. With promising development of PETase enzymes of greater efficiency under moderate temperatures and applicability to a broader range of plastic types, the future of PET is severely reduced. Even so, several industrial challenges hinder PDEs’ widespread implementation. Specifically, the efficiency of these enzymes in degrading plastics at a commercially viable rate is the primary concern. Çakar et al. call for the development of enzyme stability, durability, and “feasibility of implementing enzyme biocatalysis on a large scale” (480). Another difficulty arises from the need for widespread production methods. While some plastic-eating enzymes are naturally produced by microorganisms, others may require directed evolution technology, such as the synthetically developed FAST-PETase. To overcome both these hurdles, further research and communication are essential. Ultimately, collaboration with perspectives from biochemical researchers, policymakers, and environmental organizations for proper funding and innovation is crucial in this race to eliminate the pervasion of plastic pollution in the 21st century.
Works Cited
Anuar, Khairul, et al. “An Overview into Polyethylene Terephthalate (PET) Hydrolases and Efforts in Tailoring Enzymes for Improved Plastic Degradation.” International Journal of Molecular Sciences, vol. 23, no. 20, Oct. 2022, pp. 1-25. EBSCOhost,
https://doi.org/10.3390/ijms232012644.
Barclay, Amelia, and K. Ravi Acharya. “Engineering Plastic Eating Enzymes Using Structural Biology.” Biomolecules, vol. 13, no. 9, Sept. 2023, pp. 1-11. EBSCOhost, https://doi.org/10.3390/biom13091407.
Çakar, M. M., et al. “Discovery of Plastics-Degrading Enzymes.” Kemija u Industriji, vol. 72, no. 7/8, July 2023, pp. 473–85. EBSCOhost, https://doi.org/10.15255/KUI.2022.076. Deng, Binyang, et al. “Improving the Activity and Thermostability of PETase From Ideonella Sakaiensis Through Modulating Its Post-Translational Glycan Modification.” Communications Biology, vol. 6, no. 1, Jan. 2023, pp. 1-10.
https://doi.org/10.1038/s42003-023-04413-0.
Lee, Ga Hyun, et al. “Biotechnological Plastic Degradation and Valorization Using Systems Metabolic Engineering.” International Journal of Molecular Sciences, vol. 24, no. 20, Oct. 2023, pp. 1-25. EBSCOhost, https://doi.org/10.3390/ijms242015181.
Orlando, Marco, et al. “Microbial Enzyme Biotechnology to Reach Plastic Waste Circularity: Current Status, Problems and Perspectives.” International Journal of Molecular Sciences, vol. 24, no. 4, Feb. 2023, pp. 1-36. EBSCOhost, https://doi.org/10.3390/ijms24043877.
Patel, Sanjay K. S., and Jung-Kul Lee. “Plastic Eating Enzymes: A Step Towards Sustainability.” Indian Journal of Microbiology, vol. 62, no. 4, Dec. 2022, pp. 658–61. EBSCOhost, https://doi.org/10.1007/s12088-022-01041-w.
7
Sevilla, Maria Eduarda, et al. “Degradation of PET Bottles by an Engineered Ideonella Sakaiensis PETase.” Polymers, vol. 15, no. 7, Apr. 2023, pp. 1-15. EBSCOhost, https://doi.org/10.3390/polym15071779.
TED-Ed. “The Smallest Solution to One of Our Biggest Problems – Tierney Thys and Christian Sardet.” YouTube, 7 July 2022, www.youtube.com/watch?v=-m0YaE8uKcg. Accessed 2 Feb. 2024.
“Topic: Global Plastic Waste.” Statista, 10 Jan. 2024,
www.statista.com/topics/5401/global-plastic-waste/#topicOverview%20https://www.scie ncedirect.com/topics/earth-and-planetary-sciences/polyethylene-terephthalate#:~:text=PE T%20is%20widely%20used%20to,resistance%20and%20excellent%20dimensional%20s tability.
Wei, Ren, and Wolfgang Zimmermann. “Microbial Enzymes for the Recycling of Recalcitrant Petroleum-Based Plastics: How Far Are We?” Microbial Biotechnology, vol. 10, no. 6, Nov. 2017, pp. 1308–22. EBSCOhost, https://doi.org/10.1111/1751-7915.12710.
Yoshida, Shosuke, et al. “A Bacterium That Degrades and Assimilates Poly(Ethylene Terephthalate).” Science, vol. 351, no. 6278, Mar. 2016, pp. 1196–99.
https://doi.org/10.1126/science.aad6359.